Carbohydrates, also known as hydrated carbons are organic compounds. They contain hydrogen and oxygen in the same ratio as in water plus carbons. Therefore they are called hydrated carbons or carbohydrates. They are also called carbs (short form). These carbs are very important for human body as they provide us the basic energy needed for different routine works. The metabolism of its basic unit that is called glucose yield energy in the form of ATPs (which is called the energy currency of body).
Now if you are a medical student then you would be interested in determining these carbohydrates in a solution. This determination could be of two types. One is called qualitative analysis in which we try to find whether carbohydrates are present in the solution or not. And the other one is called quantitative analysis in which we find the amount of carbohydrates present in a given solution.
For qualitative analysis, we have already written a post on molisch’s test. Which is a general test for carbohydrates.
But today, we are going to discuss anthrone test which also a general test for carbohydrates but it is for both qualitative as well as quantitative analysis of carbohydrates.
Anthrone Method:
As mentioned above, this method can be used for quantitaive analysis which means that you could find the amount of carbs present in a given solution. This test is somewhat same as molisch test but here you can find the absorbance using photoelectric colorimeter or spectrophotometer.
Principle Of Anthrone test:
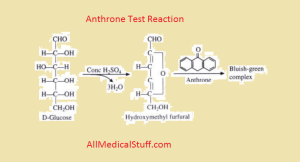
Its principle is much same as molisch test but here anthrone reagent is used instead of molisch reagent which gives green colored complex. The intensity of which is directly proportional to amount of carbs present in given solution.
First carbohydrates are hydrolyzed by treating with acid to form furfurals and hydroxy-methly furfural. These furfural are then condensed by anthrone reagent to form a blue green color complex. The intensity of which can easily be measured with 620-630nm using photo colorimeter or spectopotometer. This absorbance can then be used to calculation the amount of carbohydrates using beer- lambert law.
Anthrone Reagent:
Here is the list of reagents that are used for this test:
- Original solution that is to be tested.
- Anthrone reagent.
- H2SO4
- Standard glucose solution.

Anthrone reagent preparation:
This reagent can be prepared by dissolving 200mg of anthrone reagent in 100ml of conc sulphuric acid.
Standard Glucose Solution:
- Stock standard: Add 100mg of glucose in 100ml of H2O.
- Working Standard: Take a volumetric flask, take 10ml of stock in it and dilute with 100ml of distilled water.
Procedure of Anthrone method:
Procedure of this test is quite simple and similar to most of the other quantitative tests.
- Prepare standard solution.
- Take 6 test tubes.
- In the first one don’t add anything. In the second, add 0.2ml of standard solution, in third 0.4ml, in 4th 0.6 ml and so on respectively so that 0.2-1ml of solution is added in five test tubes.
- Then add water to all the test tubes to make it to the level of 1ml. This way the first one i.e blank only 1ml of water would be added (in which we haven’t added standard solution would have 1ml of water). While in that last one water would not be added (in which we have added 1ml of standard).
- Then add 4ml of anthrone reagent to all the test tubes.
- Heat all the test tubes in boiling water bath for 10 minutes.
- Then cool it rapidly.
- Color would change to blue green.
- Measure the optical density of this color at 630nm using photo colorimeter or spectrophotometer.
- Draw a graph and plot conentration of standard on x axis while absorbance on y-axix.
- You can easily calculate the amount of sugar from the graph using beer lambert law.
Precautions:
Here are some of the precautions for anthrone test:
- Weight exactly 100mg of glucose while making stock.
- Keep test tubes for 10 minutes in water bath. Use a stop watch for this purpose.
- Cool the solution rapidly after removing from water bath.
- Make all the six tubes till 1ml after adding solution.
- Place the test tubes in water bath carefully.
- Use Sulfuric acid with great care as we are using conc acid in this test and it could burn your skin.
Results:
As clear from the procedure of this test above, you can calculate the amount of sugar present in any solution using this test. After adding reagent and boiling in water bath for 10 min green color complex would be formed. This color complex would then determine the amount of sugar in given solution.
Final Words:
If you are a medical student and have this anthrone test in your practicals, this simple guide would help you to learn the principle and procedure of this test. Also this would help you complete your practical notebook.
If you have any queries, feel free to ask in the comments section below.
we did the anthrone test for carbohydrates but they yielded a cloudy gray/white solution? what is the answer to this?
have you used all the reagents i have mentioned? and boiling water bath etc? have you checked that the solution had carbohydrates or not? follow the procedure given above, i am sure it would work…
I think the cloudy gray solution would not contain carbohydrates…
Cloudy nature is due to the fact that when you were boiling fair amount of water got in your sample.
Thanks for contributing and sharing your knowledge.
If a milkiness is produced dilute carefully with glacial acetic acid or 50% concentrated sulphuric acid.
We need the antrone test but got a brown color instead of blue green….
do the test again…don’t know about brown color but i think it is negative results or you have’t performed correctly.
I tried it and the brown solution could be due to heating the solution at a too high temperature. So I suggest reducing it. I tried this and reducing the temperature to 90-degree C helps. You can give it a try and see whether it works. If not, you can try experimenting with other temperatures.
thanks for your contribution.
please sir, The condensation reaction occurring in the experiment removes which chain from the complex compound ?
look at the image above in principle of anthrone section…
what is the role of
sulfuric acid in this experiment
sorry…would update this post with answer to your question.
Sulfuric acid removes elements of water from the carbohydrates to form furfural
why do we carry out this rigorous preparation for CHO analysis? ,why the hydrolysis during the course of the analysis? why do we centrifuge and collect the supernatant? what is the content of the supernatant collected? the acid in the analysis performs two functions, mention them.
sorry. i performed this test during 1st year MBBS…by now i have forgot most of the things about anthrone test. so can’t answer such in depth questions at the moment.. as i am not a biochemist..but i will try to find answers to your questions…and would update this post with your answers.
different between anthrone and molisch’s test.
molisch test is qualitative while anthrone is quantitative.
What is the range of OD for the mentioned sugar concentration ?
sorry…i will get back to you with answer.
why is strong sulphuric acid used. can we also use HCL of the same strength, if not why
we need an acid to hydrolyze carbs to furfural and i think HCL would do the same but i am not sure about it because i haven’t tried it personally.
what is the role of sulphuric acid in anthrone and molisch’s test?
to form furfural.
don’t think anthrone is soluble in HCl
will you please tell me that how can we extract carbohydrates from leaf material or stem material of a plant. and what should be the final dilution of the sample.
sorry don’t know the exact method…you can search for it in youtube.
Can coloured solution or sample can be used for the for anthrone test
Nice work admin.
please can you give me a likely graph that would yield 20% total sugars?
nice work,
Its possible to get negative results in the working standard.
Hi
What is the difference bw alphanaphtol reagent in molisch test and anthrone reagent,what are their mechanism in the tests?
And also what happen in anthrone test which we can measure the amount of carbohydrate but in molisch we can’t measure it?
How to write a discussion for this experiment in practical report
write in the same way i have written but in your own words.
Please what is the role of sulphuric acid in both molisch and anthrone tests
i have mentioned this in the principle of molisch’s test. this hydrolyse the carbs and furfurol is formed.
I don’t understand..the result when reacted on glucose is not clear.
have you performed the test with glucose? have you followed the exact steps?
for drawing the graph of absorbance and concentration,please help me calculate the concentration if I have the absorbance